According to the Centers of Disease Control and Prevention (CDC), more than 2.8 million antibiotic-resistant infections occur in the U.S. each year, and more than 35,000 people die as a result. Worldwide drug-resistant diseases kill around 1.2 million people each year. And if nothing is done, by the year 2050, a United Nations interagency group estimates that 10 million people will die from drug-resistant diseases; a number that equals the amount of people that were killed by cancer in 2020, according to the World Health Organization.
Antibiotic resistance is a serious public health problem. Some bacteria that can cause serious disease are becoming resistant to most commonly available antibiotics. And alarmingly, antibiotic resistant bacteria can spread from person to person both in the community and from one patient to another in hospital settings.
One of the main causes of antibiotic resistance is the overprescribing of antibiotics. At least 30% of antibiotics prescribed in the United States are unnecessary, according to the CDC. There is no question that antibiotics are miracle drugs, but their overuse is killing us and leading to superbugs which are strains of bacteria that are resistant to most of the antibiotics commonly used to treat the infections they cause. A few examples of superbugs are resistant bacteria that can cause pneumonia, urinary tract infections, and skin infections.
At present, the list of bacteria that cannot be killed by standard antibiotic treatment includes methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), multi-drug-resistant Mycobacterium tuberculosis (MDR-TB) and carbapenem-resistant Enterobacteriaceae (CRE) gut bacteria.
Is the answer better than antibiotics? We do not know, because the pharmaceutical industry has abandoned looking for new antibiotics. According to an article in Nature, “fewer new antibiotics are reaching the market; the last entirely original class of antibiotic was discovered in the late 1980s.” In the last decade, Big Pharma is turning to areas such as cancer where new drugs are needed and big profits can be made, which is not the case with antibiotics.
So, if you get a superbug, what can you do? Sometimes looking backwards at how illnesses were treated in the past is the answer. At least it was for two doctors who work at the University of California San Diego School of Medicine.
When Steffanie A. Strathdee’s husband, psychologist Tom Patterson, became ill and was told he was going to die, she used her research skills and determined that phage therapy might be the answer. (Dr. Strathdee is an infectious disease epidemiologist. She is Associate Dean of Global Health Sciences at the University of California San Diego School of Medicine and co-directs UCSD’s new center for Innovative Phage Applications and Therapeutics (IPATH). Tom Patterson is a professor of psychiatry at UCSD School of Medicine.)
Ironically, Tom got ill when he and his wife were on vacation in Egypt in 2015. Dr. Strathdee said, “We were in Egypt and basically the only people on a cruise. Tom developed a stomach bug. He took an antibiotic, and we expected the discomfort to pass. Instead, his condition turned critical. We learned that Tom had contracted a life-threatening infection with a multidrug-resistant strain of Acinetobacter baumannii, which is an opportunistic and often deadly bacterium.”
Local doctors at an Egyptian clinic, an emergency medevac team, and then a German hospital failed to cure him. He was then medevaced to their university hospital at UC San Diego where their colleagues treated him. But despite everyone’s best attempts, Tom was dying. “As a last resort, I used my research skills to look at both new and old research and came across phage theory: the idea that the right virus, aka ‘the perfect predator,’ can kill even the most lethal bacteria. Phage treatment had fallen out of favor almost 100 years ago, after antibiotic use went mainstream. My colleagues and I appealed to phage researchers all over the world for help and we were able to save my husband,” Dr. Strathdee said.
Dr. Patterson became the first known person in the United States to successfully undergo intravenous bacteriophage (phage) therapy to treat a systemic superbug infection. Dr. Strathdee recounted: “The medical team received emergency approval from the Food and Drug Administration (FDA). Tom was treated intravenously with an experimental phage cocktail that specifically targeted A. baumannii. He began improving almost immediately, emerging from a months-long coma. He is now fully recovered.”
Dozens of patients at UC San Diego Health have now been treated with phages at the Center for Innovative Phage Applications and Therapeutics (IPATH), which was co-founded by Strathdee in 2018. One of these patients had a years-long chronic infection that was successfully cleared, allowing him to undergo life-saving heart transplant surgery. “In all cases, the phage treatments required emergency approval by the FDA, as no form of phage therapy is currently FDA-approved, so it can only be used in emergency, experimental, or compassionate use cases,” Dr. Strathdee said. However, the National Institutes of Health has begun funding clinical trials, one of which will launch at IPATH in 2022. After IPATH, phage therapy programs have opened at Baylor and Yale Universities, and the Mayo Clinic.
What is Phage Therapy
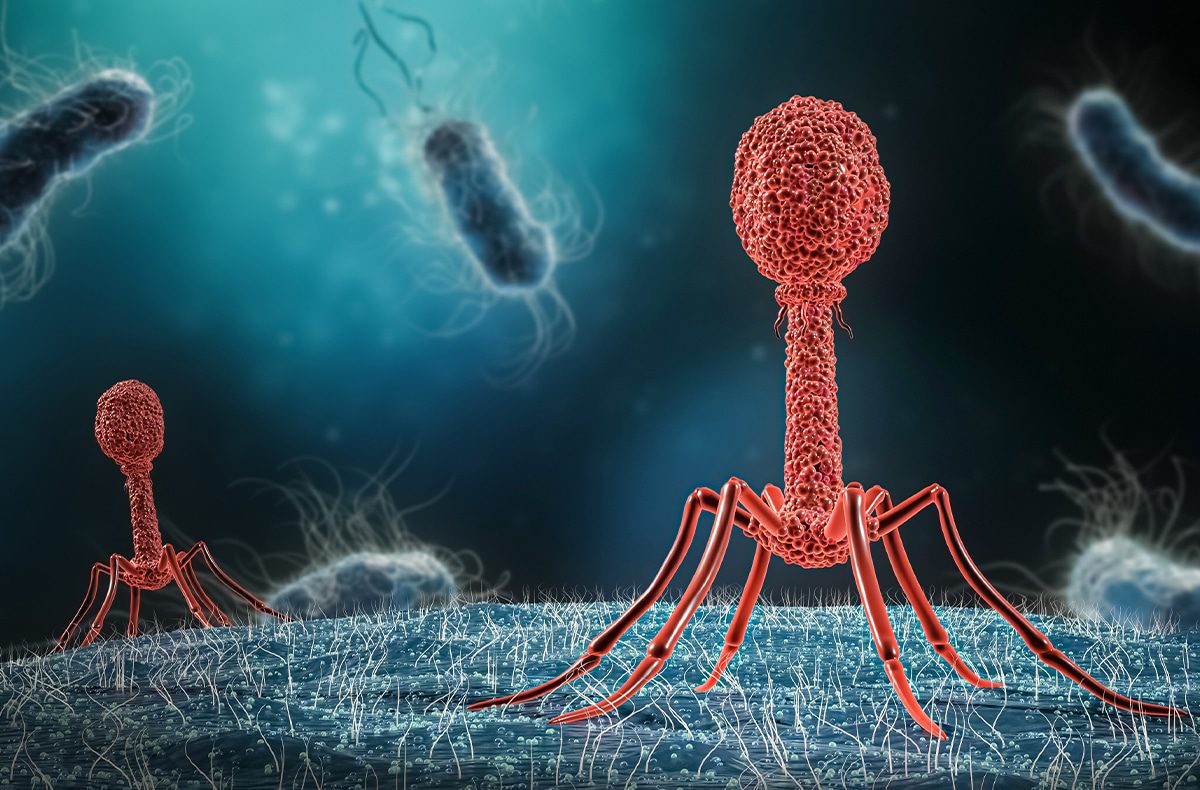
Bacteriophages, also known as phages, are ubiquitous viruses, found wherever bacteria exist. It is estimated there are more bacteriophages than every other organism on Earth combined. Phages are natural predators of bacteria, and the name “bacteriophage” translates as “eaters of bacteria.” Phages infect and rapidly kill the bacterial host by multiplying inside and then bursting through the cell membrane to release the next generation of phages into the surrounding environment, ready to infect and kill additional nearby target bacterial cells until the bacteria have been eliminated. When there are no target bacteria left for the phages to infect, the phages are eliminated through the body’s natural clearance processes. An advantage of phages is that they only attack the bacteria that have a receptor they match to, unlike many antibiotics that also kill the friendly bacteria in our microbiome.
“The use of natural and synthetic phages has several advantages compared to traditional antibacterial therapies based on the unique mechanism of action of phages. A typical life cycle of a lytic phage takes around 30-60 minutes, and only stops once the pathogenic bacteria they match have been eliminated,” explained Dr. Strathdee.
As an infectious disease specialist, I asked why she did not know much about phage therapy. “I would say the main reason is that I don’t live in Eastern Europe or Russia where phage therapy is used. In the 1920s and 30s, researchers around the world continued to study and test phages for their ability to treat bacterial infections in humans. However, most of the results of those studies were published in non-English journals and therefore did not immediately spread to Western Europe and the U.S.”
“In the 1940s, the pharmaceutical company Eli Lilly produced phages for human use in the U.S., and they were marketed to treat a range of bacterial infections, including wounds and upper respiratory infections. But it was suspected that the phages did not work all that well, partly since they were improperly stored or purified, and it was not recognized at the time that many phages were highly selective about the kind of bacteria they infected. Phage therapy fell out of favor in the U.S. and most of Europe with the advent of antibiotics. Only in regions where antibiotics were not as easily accessed—namely what is now Russia, Poland, and the Republic of Georgia—did phage therapy and commercial production continue. However, the phage studies conducted in these regions continued to be non-randomized and uncontrolled, and thus empirical data is still lacking to show that phage therapy was effective.”
To learn more about this fascinating treatment and its yet untapped potential, you can read Dr. Strathdee’s and Patterson’s story in their book The Perfect Predator, A Scientist’s Race to Save Her Husband from a Deadly Superbug: A Memoir, and watch the full interview I did with Dr. Strathdee for Science Writers in New York here.